The Entropy Of An Isolated System
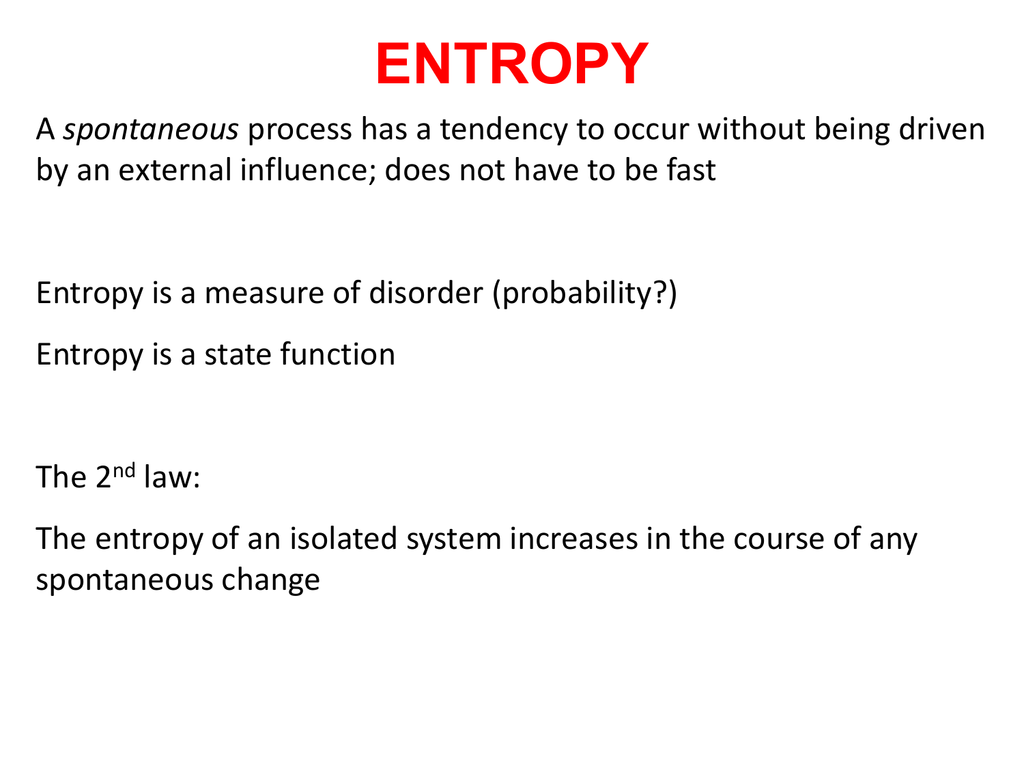
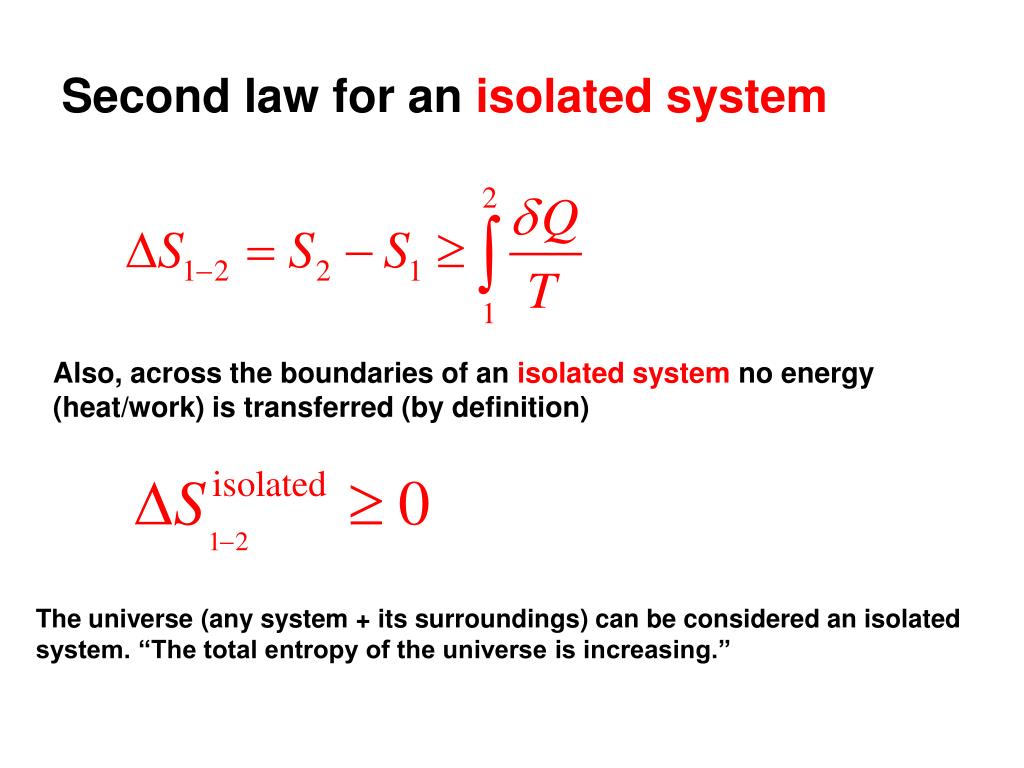
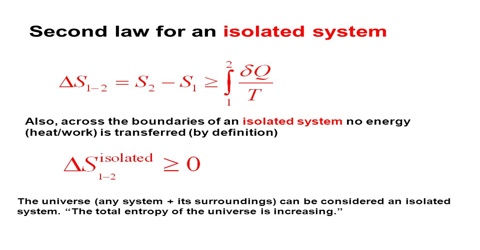
The entropy of an isolated system. In an isolated system natural processes are spontaneous when they lead to an increase in disorder or entropy. Changes in physical state and entropy changes During the phase transition the temperature remains constant At the temperature of phase transition the transfer of heat is reversible. The second law of thermodynamics states that any isolated systems entropy always increases.
Every system left to itself will on the average change toward a condition of maximum probability. Allows you to buy electricity at a lower rate from the provider b. That is why computers will always need cooling systems.
This preview shows page 2 - 3 out of 3 pages. All what Im really saying is that the room as whole is not at equilibrium meaning that the system is exchanging heat. The entropy change of a system or its surroundings can be negative.
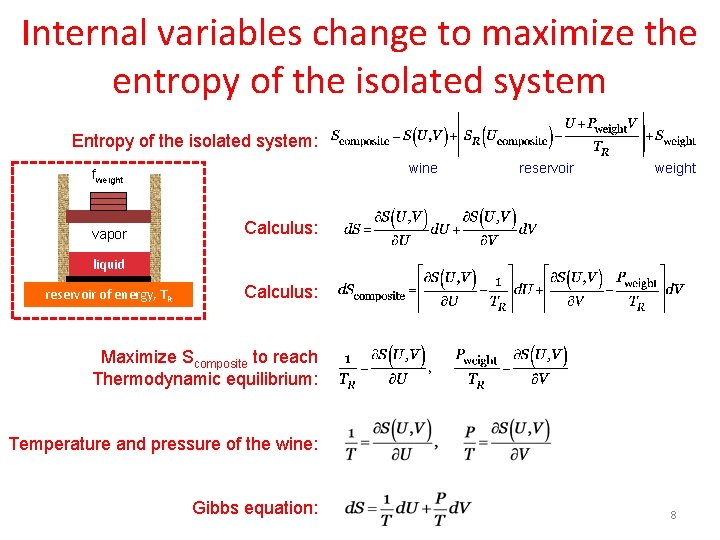
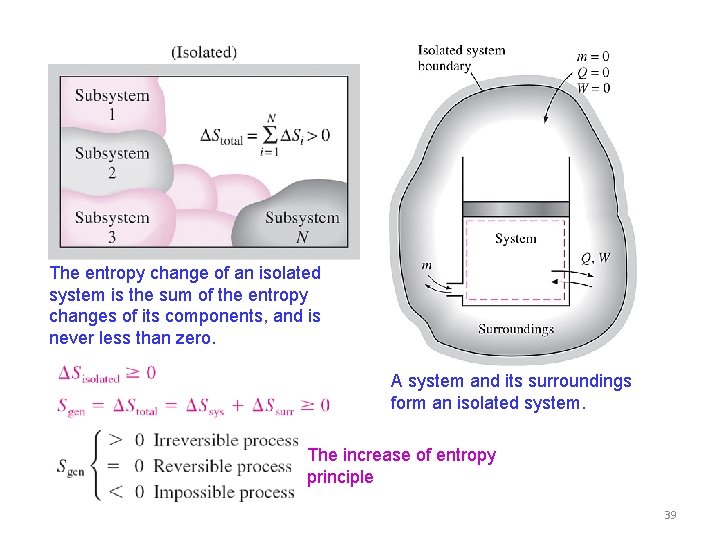
The universe is an isolated system if we consider the universe to be everything then there is nothing for it to exchange matter andor energy with. Statement I - The entropy isolated system with P V work only is always a maximized at equilibrium. Since we know that.
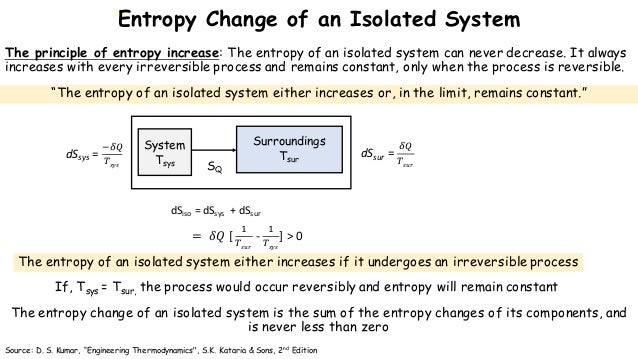
In simple terms Universe entropy the ultimate isolated system only increases and never decreases. This means once maximum entropy is reached the entropy will no longer increase as it has already reached maximum value. The sum of entropy change in system and entropy change in the surrounding is.
This statement is restricted to isolated systems to avoid having to worry about whether the reaction is exothermic or endothermic. Second law of thermodynamics. An isolated system is such that matter and energy cannot enter or exit the system.
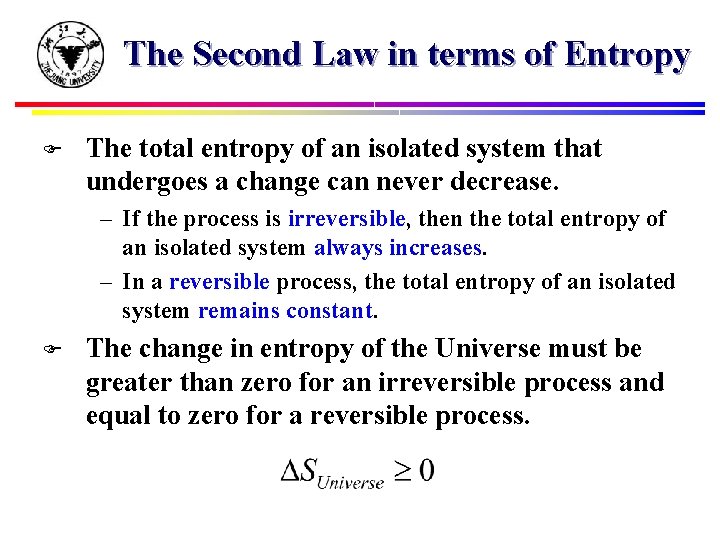
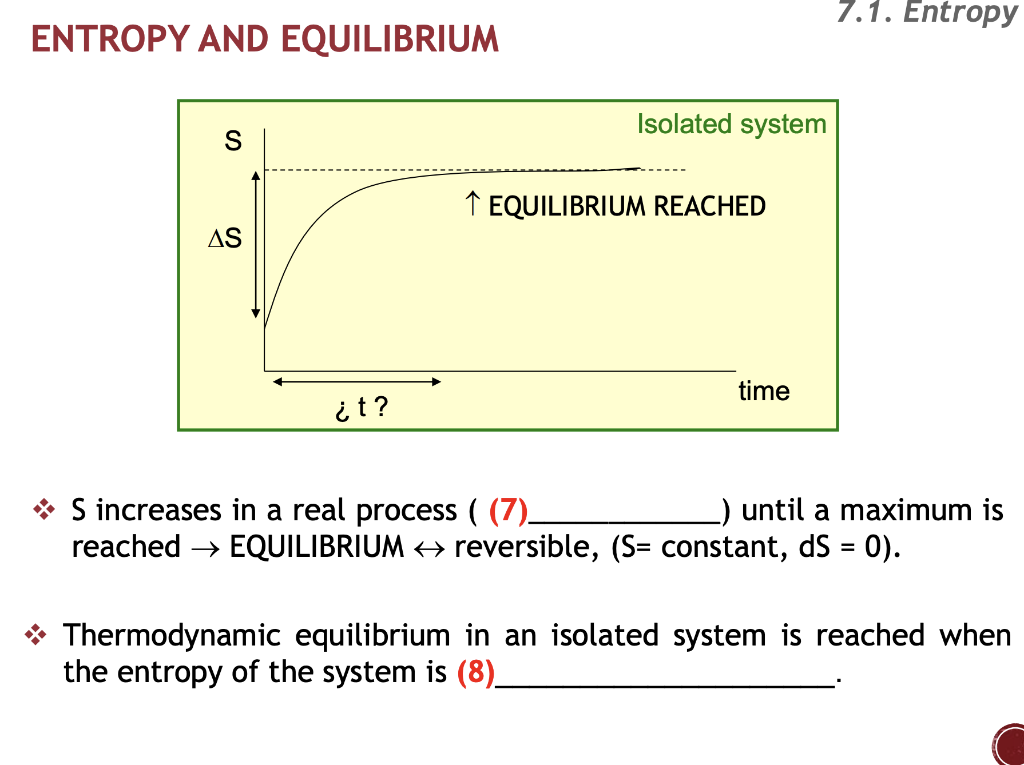
The entropy of an isolated system increases only until it reaches a maximum possible value. The measure of disorderly dispersal.
If during a process the entropy of the isolated system increases the process is.
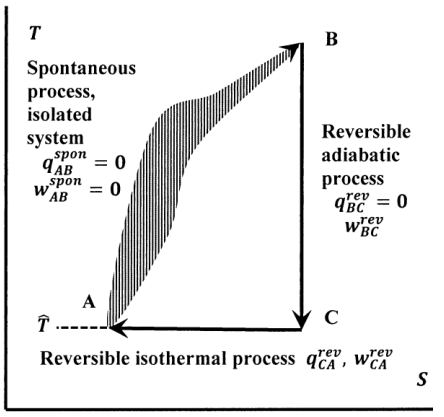
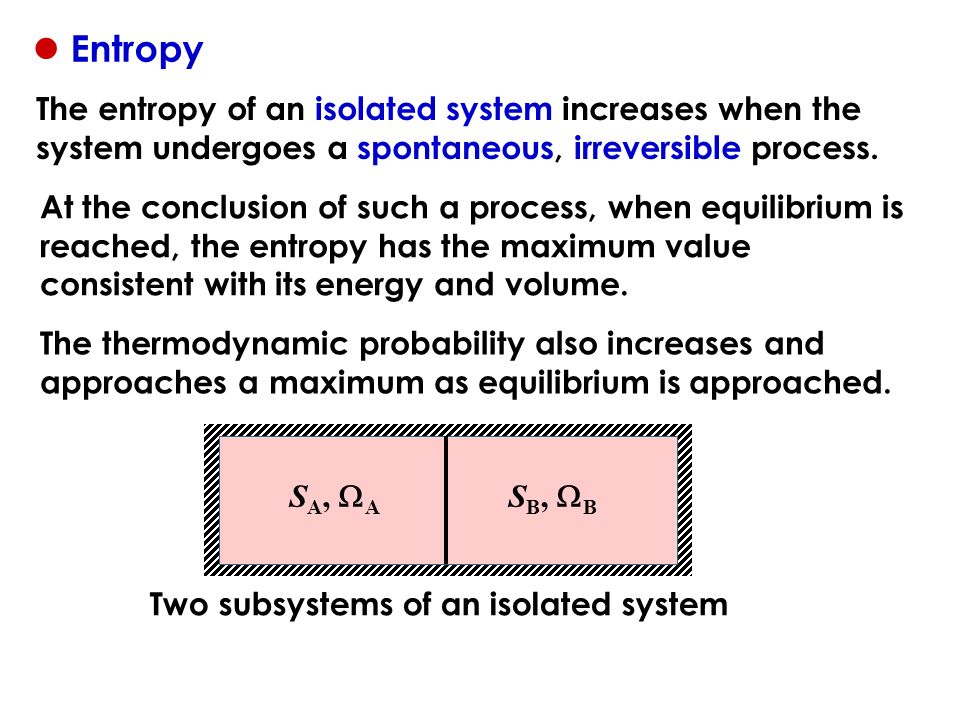
In that case when the systems entropy reaches the maximum the system stays there because any further change would reduce entropy. In an isolated system natural processes are spontaneous when they lead to an increase in disorder or entropy. This may seem like a special case but its not. The entropy of an isolated system increases only until it reaches a maximum possible value. Moreover all real life processes are irreversible so for any process AB occurring in the universe the entropy of. All what Im really saying is that the room as whole is not at equilibrium meaning that the system is exchanging heat. Changes in physical state and entropy changes During the phase transition the temperature remains constant At the temperature of phase transition the transfer of heat is reversible. An example of entropy production in an isolated system as system that exchanges neither heat work or mass with its surroundings is the irreversible expansion of a gas into a vacuum in an insulated rigid vessel. Isolated systems evolve spontaneously towards thermal equilibrium the systems state of maximum entropy.
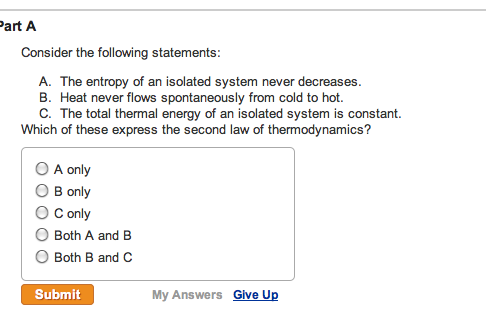
The entropy of an isolated system not in equilibrium will tend to increase over time approaching a maximum value at equilibrium. Every system left to itself will on the average change toward a condition of maximum probability. In other words The second law of thermodynamics states that the total entropy of an isolated system always increases over time or remains constant in ideal cases where the system is in a steady-state or undergoing a reversible process. Second law of thermodynamics. The measure of disorderly dispersal. Allows you to buy electricity at a lower rate from the provider b. Let be the number of accessible microstates.































Post a Comment for "The Entropy Of An Isolated System"